Check our 3-Way Catalytic Converters designed especially for gasoline and gaseous LSI and SSI engines
How Does a Three-Way Catalyst Work?
In the three-way catalyst, reactions between CO, HC, and NOxresult in the simultaneous removal of all three major exhaust pollutants. One of the fundamental chemical reactions occurring in the three-way catalyst can be written as follows:
CO + NO = CO2 + 1/2 N2
Concentrations of pollutants in the exhaust gas depend on the fuel mixture composition. At lean fuel mixtures the exhaust gases contain little carbon monoxide or hydrocarbons but high concentrations of NOx. Rich mixtures produce high concentrations of CO and HC with little NOx. In order to achieve high simultaneous conversions of CO and NOx, their concentrations in the exhaust must be in stoichiometric proportion, as illustrated by the above equation. If the air-to-fuel mixture is not stoichiometric, conversion of either NOx or CO will deteriorate, as shown in Figure 9.
In practice, three-way catalysts are used with air to fuel ratio controllers which maintain the mixture composition at stoichiometric. These controllers utilize a feedback signal from the oxygen sensor positioned in front of the catalyst in the exhaust system. The range of A/F ratio required for satisfactory catalyst operation is known as the "catalyst window". Precise A/F ratio control is especially important for efficient NOx control, as the nitrogen oxides conversion drops dramatically at lean fuel mixtures.
Nett Technologies’ 3-Way Catalytic Converters

Request A Quote
Photo Gallery

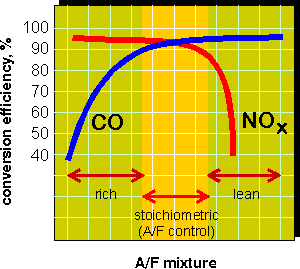 Figure 9. Three-Way Catalyst Performance
Figure 9. Three-Way Catalyst Performance